Abstract
Introduction:
Daratumumab (DARA) is a first-in-class, fully human IgG1 kappa MoAb that targets to a unique CD38 epitope in plasma cells and has been recently approved for patients with relapsed and refractory myeloma multiple (RRMM) that have received ≥3 prior lines of therapy including proteasome (IP) inhibitor and an immunomodulatory agent (IMiD). Based on pharmacokinetic analysis it is not necessary to adjust the dosage of DARA in patients with creatinine clearance 30-60 mL/min. However there are no published data available in patients with RRMM requiring dialysis.
Aims:
The objective of this retrospective, multicenter, open-label study is to report the safety and efficacy of DARA in patients with RRMM with end-stage renal failure requiring dialysis.
Methods:
The eligibility criteria to participate in this study included patients ≥18 years, documented progression of MM by IMWG criteria following the most recent therapy including a PI and an IMID or double-refractory to a PI and an IMID, ECOG performance status score 0-2, absence of chronic obstructive pulmonary disease or persistent asthma, and no prior exposure to anti-CD38 antibody therapy. Patients received DARA at the standard dose (16 mg/kg IV weekly for 8 weeks, then every 2 weeks for 16 weeks, and then every 4 weeks) until disease progression or unacceptable toxicity. Pre-medications with antihistamines, antipyretics and corticosteroids and post-infusion medications with corticosteroids were administered including the use of montelukast. Anti-viral prophylaxis was considered. Data on anti-MM activity and adverse events (AEs) including grade 3-4 AEs and infusion related reactions (IRRs) were collected.
Results:
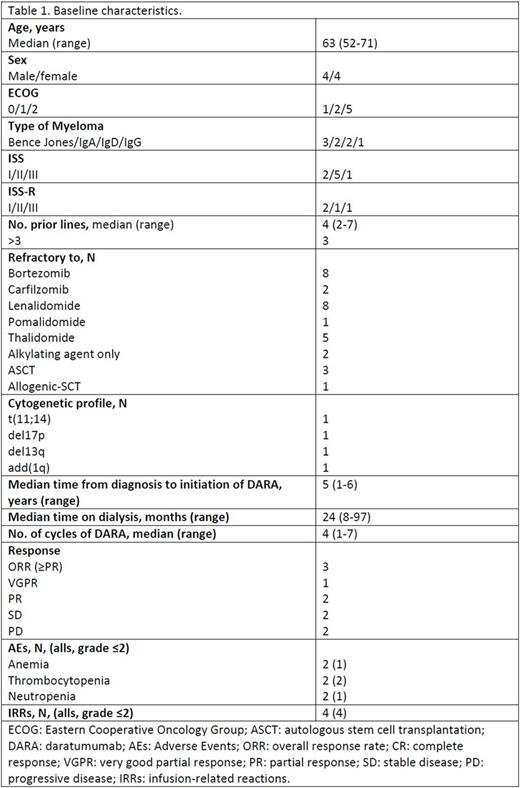
The study, including eight Spanish centers is ongoing and we report here the preliminary results of the first eight patients included from January 2017 to July, 2017. There were 4 male and 4 female with a median age at the time of the beginning of DARA therapy of 63 years (range, 52 -71) and with a median time from diagnosis to initiation of DARA of 4.61 years (range, 1 - 6). MM was Bence Jones in 3 patients, IgA type in 2, IgD in 2, and IgG in 1 patient. Distribution of disease according to the International Stage System was: 5 patients were in stage II, 2 patients were in stage I, and the remaining patient was in stage III. The median number (range) of prior lines of therapy was 4 (2 -7); 37% of patients received >3 prior lines. All patients were double refractory to both a PI and IMiD, 2 patients were also refractory to carfilzomib, and 1 patient was triple refractory including pomalidomide. Four patients had received previous stem-cell transplant (3 autologous and 1 allogeneic).
At the time of diagnosis, 2 out of the 8 patients had high-risk cytogenetics [1 patient with add(1q) and 1 with del(17p)]. Detailed patient characteristics with respect to baseline clinical features and laboratory parameters are provided in Table 1. In 6 patients, end-stage renal failure was myeloma-related (2 patients with acute renal injury at time of diagnosis), while the remaining 2 patients were already on dialysis at the time of MM diagnosis. The median time from the diagnosis of MM and the beginning of the dialysis was 5 years (range, 1-6). The median time on dialysis at study entry was 24 months (range, 8-97).
The median (range) number of cycles of DARA administered was 4 (1-7). The overall response rate and the clinical benefit rate (stable disease or better) was 37.5% (1 VGPR and 2 PR) and 62.5%, respectively. No patient has become dialysis independent.
With a median follow-up of 4 months, 2 patients have died because of disease progression (1 patient) and due to myeloma-related causes (1 patient) and one patient suffered disease progression. The remaining 5 patients are still alive with ongoing DARA treatment. Regarding safety, no patient discontinued treatment due to AEs and the most common non-hematological AEs included grade 1 or 2 IRRs observed only during the first cycle, (4 patients). Anemia (n=2, grade 2 and 3), thrombocytopenia (n=2, grade 1 and 2), and neutropenia (n=2, grade 1 and 4), were the hematological AEs most frequently observed.
Conclusions:
Although still preliminary, our data suggest that DARA monotherapy is an efficacious and safe therapeutic option for patients with RRMM and end-stage renal failure including dialysis. AE profile seems to be similar to that previously observed in patients with normal or moderately impaired renal function.
de la Rubia: Celgene: Other: Honoraria; Janssen: Other: Honoraria; Amgen: Other: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal